The University of California, Santa Cruz, has high standards for faculty conduct, including the conduct of research, as discussed in the University of California Faculty Handbook and the Academic Personnel Policy Manual. All UC Santa Cruz investigators are expected to carry out research consistent with these standards. UC Santa Cruz as an institution is also expected to uphold UCOP’s Institutional Conflicts of Interest in Research principles.
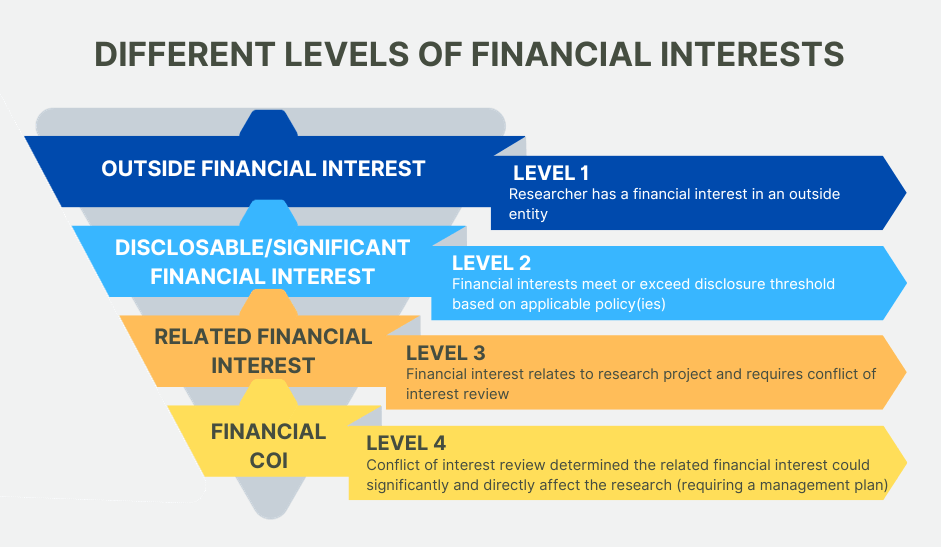
Even when these high standards have been met, conflicts of interest or perceptions of conflicts may still occur when an investigator’s private interests converge with the investigator’s research interests. In these cases an independent observer might reasonably question whether the investigator’s professional actions or decisions are improperly influenced by considerations of personal financial gain. Such conflicts are common in modern research universities.
The university requires disclosure of personal interests for extramural funding. Such disclosure processes are the most widely accepted method for identifying and managing actual or potential conflicts of interest related to sponsored projects in public institutions. The Conflict of Interest Review Committee (COI Committee) has been established to comply with regulations that require review of such potential conflicts. When the COI Committee determines that an interest could directly and significantly affect the sponsored project, the university will take steps to manage or to remove the conflict.
Conflict of interest training (ECBR)
Conflict of interest definitions
| Term | Definition |
|---|---|
| Investigator | The project director (PD), principal investigator (PI) and any other person, regardless of title or position, who is responsible for the design, conduct, or reporting of the funded research. |
| Financial interest | Anything of monetary value, whether or not the value is readily ascertainable. |
| Significant Financial Interest (SFI) | Anything of monetary value, including, but not limited to, salary or other payments for services (e.g., consulting fees or honoraria); equity interest (e.g., stocks, stock options, or other ownership interests); and intellectual property rights (e.g., patents, copyrights, and royalties from such rights). See specific sponsor requirements for details and thresholds. |
| Conflict of Commitment (COC) | COC arises when an individual’s participation in outside professional activities interferes with their professional obligations to the university. UCOP governs COC requirements under APM-025, and faculty COC disclosures are managed by the Academic Personnel Office through the Outside Activities Tracking System (OATS). Faculty and other academic appointments in key personnel roles on sponsored projects are also responsible for providing annual and/or project-based conflict of commitment disclosures, as required by the terms and conditions of their awards. |
Sponsor-specific disclosure guidance
Disclosure process
The Office of Sponsored Projects, IBE Hub, or Gift Administration will prompt you to submit a UC Santa Cruz Disclosure when needed (e.g., you are receiving support from a grant, contract or gift for research). ORCA will review your submission and reach out with next steps, if needed. If your financial interests change after submission of a disclosure or additional investigators are added, you are required to resubmit your disclosure.